

 Chapter 7 Electronic Structure of Atoms
Chapter 7 Electronic Structure of Atoms
-
College Level - Everything there is to know about physics in a hyper-linked site. This is heavy duty information in a logical organization.
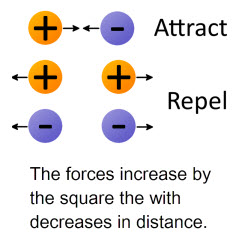
Electrostatic (Coulombic) attractions and repulsions are the predominate force on the atomic scale in chemistry. Coulombic forces are important in explaining the electronic structure of the atom and ionic and molecular behaviors.
 .
.
Use the link to find the surprising Coulombic answer to the question and also why Elspeth is in a Faraday cage.

Guest Lecturers: Rob Lederer and Mystery Lecturer
 Orbital Shape
Orbital Shape Electron Configuration I
Electron Configuration I  Whiteboard
Whiteboard Electron Configuration II
Electron Configuration II  Whiteboard
Whiteboard Electron Configuration of bromine (and valence electron counts)
Electron Configuration of bromine (and valence electron counts)- Except for the Primary Energy Level number, quantum numbers are not in the AP Curriculum.
- Yet, Rob Lederer's explanations of the Quantum numbers are worth your time since the numbers
show the patterns of electronic structure and are on the CLEP-Chemistry Subject Test.  Quantum numbers 1 - codifying electron properties in an atom
Quantum numbers 1 - codifying electron properties in an atom Quantum numbers 2 - addresses of electrons in a lithium atom
Quantum numbers 2 - addresses of electrons in a lithium atom

 AP07.10 C07 Electronic Structure of Atoms
AP07.10 C07 Electronic Structure of Atoms

Take the FRQ Quanta Quiz on paper first and use the scoring guide to grade yourself. Then, take the quiz in WebAssign.
AP07.20 C07 FRQ Quanta Quiz Scoring guide

Labs that should be completed by the end of October
 APLab.10 Lab Safety
APLab.10 Lab Safety APLab.15 How Accurate are Volume Measurements?
APLab.15 How Accurate are Volume Measurements? APLab.20 Percent Carbon in Sodium Hydrogen Carbonate
APLab.20 Percent Carbon in Sodium Hydrogen Carbonate APLab.25 Decomposition Stoichiometry
APLab.25 Decomposition Stoichiometry
