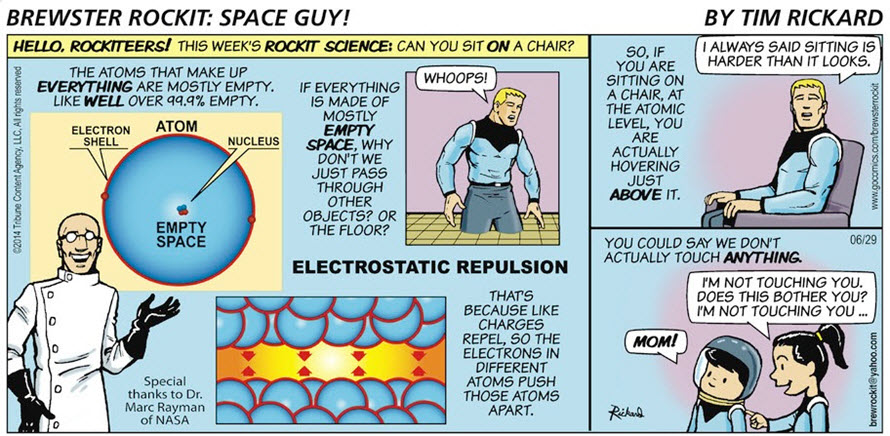
Brewster Rocket's explanation of electrostatic repulsion is correct. However the Pauli exclusion principle comes into play also.
Coulombic attractions can also hold atoms, molecules and solids and liquids together.
If Marie had a substance with half-filled orbitals on her finger she could be covalently bonded to Albert.
We will be referring to electrostatic attractions throughout the year. You should understand these forces and the inverse square law. If you need some review of Coulombic forces view the first few minutes of a lecture by Professor Eric Rogers. His emphasis of the importance of Coulombic charges is worth remembering.
Optional: The entire film has a cult following at Princeton where Eric taught for many years. The rest of the video applies to physics more than chemistry and won't apply to AP Chem.
In it Eric Rogers, continually removes and replaces his eyeglasses, and orders around lab assistants as he explains the law to us. Just be glad you are not the assistant's daughter, Elspeth,locked in the Faraday cage at the end of the full video.
Full Video: Coulombs Law 30 minutes

 .
.