Chapter 5 Gases
You know how to find moles from the mass of a substance (s) or (l) or from the concentration of a solution (aq). Next, you will learn how to find moles from the pressure, volume and temperature of a gas (g).
For those of you who like advanced math, statistics, and the Maxwell-Boltzmann curve, here is an excel spreadsheet that allows you to make your own curves. (Optional)

 AP05.10 C05 Gases I
AP05.10 C05 Gases I  AP05.20 C05 Gases II
AP05.20 C05 Gases II

Guest Lecturer: Rob Lederer
Since the AP Chemistry exam uses atm and mm Hg, I have put the white-board notes in atm and mm Hg (torr).The AP Chemistry exam and most US chemists still cling to the units torr and atm. The SI unit for pressure used by the rest of the world is the pascal. Rob Lederer is in Canada where the metric unit for pressure, kPa, is used.
Rob identifies the laws by their discoverer's names. There is no need to memorize the names as the names of the laws are not tested on the AP Chem exam.
 Gas Units
Gas Units

 Pressure Volume
Pressure Volume

-
 Temperature and Pressure and Volume
Temperature and Pressure and Volume

 Ideal Gas Law - P V = n R T
Ideal Gas Law - P V = n R T

 Gas Pressure and Molecular Collisions
Gas Pressure and Molecular Collisions

- Gas Pressure and Molecular collisions is a classic video.
- Don't be distracted by its age. I have not seen a better video explaining gas pressure.
- James Arthur Campbell, the demonstrator for the video worked on the Manhattan Project and was one of my chemistry professors.
The key developer of these the Chem Study films, Glenn Seaborg, was the only person to have had an element named after him while he was still alive. I have his autographed book on teaching chemistry.
-
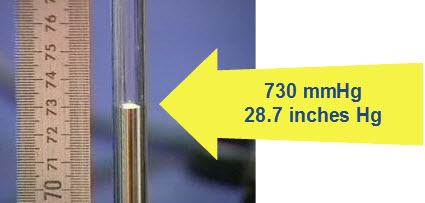
Mercury barometers are no longer because of mercury fumes. However mm Hg is still used as the unit for pressure in the US. This is how a mercury barometer is made. Watching this will help you understand air pressure and mm Hg better.

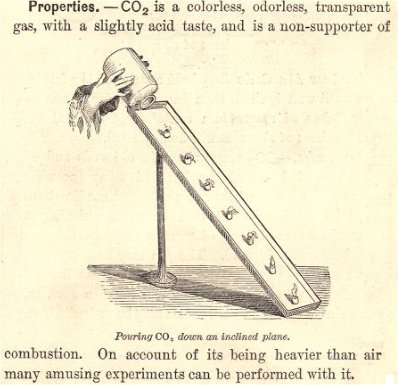
This demonstration is from my favorite chemistry book, Fourteen Weeks in Chemistry by J. D. Steele, the first American homeschool chemistry teacher.



Make sure your mental math skills are better than Calvin's.
-
 AP05.30 C05 MC Test Answer Sheet
AP05.30 C05 MC Test Answer Sheet  AP05.40 C05 MC Test Redux
AP05.40 C05 MC Test Redux

Labs that should be completed by the end of October
 APLab.15 How Accurate are Volume Measurements?
APLab.15 How Accurate are Volume Measurements? APLab.20 Percent Carbon in Sodium Hydrogen Carbonate
APLab.20 Percent Carbon in Sodium Hydrogen Carbonate APLab.25 Decomposition Stoichiometry
APLab.25 Decomposition Stoichiometry