AP11 Physical Properties of Solution
Last assignments for Semester 1
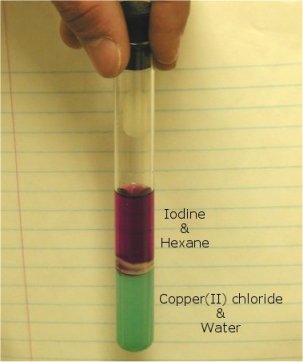
Nonpolar iodine dissolves in nonpolar hexane.
Polar water can dissolve ionic copper(II) chloride.
Chapter 12 - Physical Properties of Solutions
Demonstrations
 |
|
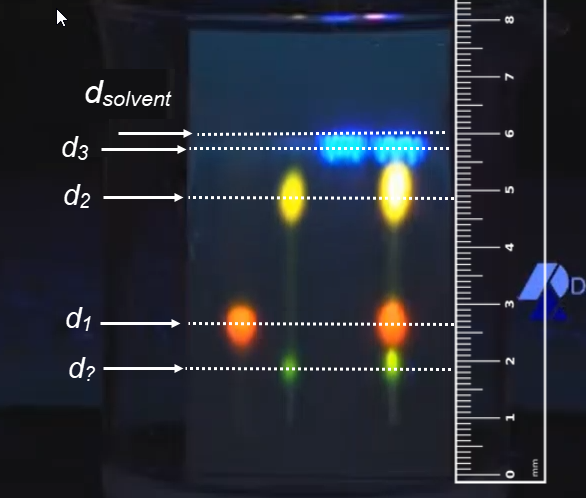 |
This important video shows the two types of chromatography that you are expected to understand: Paper chromatography and column chromatography. The video uses Thin Layer Chromatography, TLC, which uses a thin layer of gel on a glass plate rather than paper as the stationary phase. Other than that, the procedure is identical to paper chromatography. ChemToddler has a number of other excellent chemistry videos. |
 |
A video showing dipole-ion dissolving. View it a couple of times. Note which ions attract the oxygen end of the water molecules and which ions are cloaked by the hydrogens of a water molecule. Understand that the ion-ion bond is being broken as the dipole-ion intermolecular attractions are being formed. It takes quite a few water molecules to cloak a singe ion. |
 |
A Capella Science has put a video together that sums up much of what you have learned in Chapters 7,8,9,10,11, and 24 in 4 minutes and 44 seconds of song. You should understand 90% of it. The better you understand these chapters the more you'll see in the video. The only major concept that we did not cover was molecular obitals which have the up down animations. Molecular Shape of You goes from microscopic electrons, protons, atoms, molecules, and polymers to macroscopic you. |

Optional video
Molality and % concentration are not AP Chem material, but are found on the CLEP Chemistry Exam.

 AP11.10 C12 Solutions
AP11.10 C12 Solutions

Last Semester 1 Test
AP11.15 C11-C12 MC Test
AP11.25 C11-C12 MC Redux
AP11.20 C11-C12 FRQ Hybrid

Labs due Week 20, the end of the semester.
 APLab.10 Lab Safety
APLab.10 Lab Safety APLab.15 How Accurate are Volume Measurements?
APLab.15 How Accurate are Volume Measurements? APLab.20 Percent Carbon in Sodium Hydrogen Carbonate
APLab.20 Percent Carbon in Sodium Hydrogen Carbonate APLab.25 Decomposition Stoichiometry
APLab.25 Decomposition Stoichiometry APLab.30 Electrolytes
APLab.30 Electrolytes APLab.35 Metathesis Ppt Reactions
APLab.35 Metathesis Ppt Reactions  APLab.30 Electrolytes
APLab.30 Electrolytes  APLab.35 Metathesis Reactions
APLab.35 Metathesis Reactions APLab.40 Heat from a Microwave
APLab.40 Heat from a Microwave APLab.45 Specific Heat and Metals
APLab.45 Specific Heat and Metals APLab.50 Enthalpy of Solution
APLab.50 Enthalpy of Solution APLab.55 Separting a Solution
APLab.55 Separting a Solution

