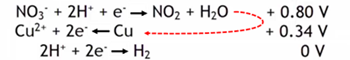
Copper will not oxidize when exposed to 1 M strong acids since its reduction potential 0.80 V is greater than the reduction potential of H+.
Copper ions would even oxidize hydrogen gas to reform copper metal.
Cu(s) + 2 H+(aq) ← Cu2+(aq) + 2 H2(g)
However nitric acid can oxidize copper metal.
Nitric acid is a strong acid, but in addition its nitrate ions in an acidic environment have a higher reduction potential than hydrogen ions and can oxidize with metals that regular acids can't.
Nitric acid can even oxidize copper as the nitrogen in the nitrate reduces from N+5 to N+4.
To balance the electrons in the reaction there must be twice as many nitrate reductions as copper oxidation reactions.
Cu(s) + 4 H+(aq) + 2 NO3-(aq) → Cu2+(aq) + 2 NO2(g) + 2 H2O(l)
Nitrogen dioxide is quite poisonous. Nitric acid spills on metals will produce this deadly gas. Nitric acid must be stored separately from other acids because of its special reactivity.