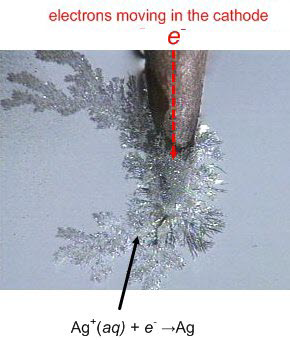
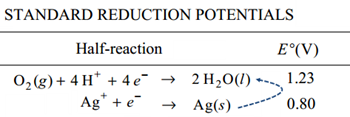The graphite rods in two pencils is hooked up to a 9 V battery and immersed in a silver nitrate solution.
.
The battery forces the oxidation of the water into oxygen and acid as the silver ions in solution gain electrons reducing from ions in solution into metallic silver crystals.
Note the voltage of this reaction would benegative and is not thermodynamically favored. The reaction is forced by the thermodynamically favored reaction in the battery.
Balanced:
(oxidation) 2 H2O(l) → O2(g) + 4 H+(aq) + 4 e-
(reduction) 4 Ag+(aq) + 4 e- → 4 Ag(s)