This solution contains cobalt(II) complexed with chloride ions in an ethanol solution.
 |
↔ |
 |
[Co(H2O)6]2+(alc) + 4 Cl- (aq)
|
↔ |
CoCl42-(alc) + 6 H2O(l) |
cobalt(II)-aqua complex |
↔ |
cobalt(II)-chloro complex |
Changes is temperature will change the equilibrium constant.
An increase in temperature, increases the equilibrium constant shifting the equilbirium → of any endothermic reaction.
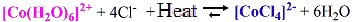
Note!
This is a solution made using alcohol (alc). The concentration of the water in the alcohol solution can change and as a result, water would be part of the equilibrium expression.
Adding water would would increase [H2O] in the (alc) causing a Le Châtelier shift ← to form more reactants.
An increase in temperature, increases the equilibrium constant shifting the equilbirium → of any endothermic reaction.
Note!
This is a solution made using alcohol (alc). The concentration of the water in the alcohol solution can change and as a result, water would be part of the equilibrium expression.
[Co(H2O)6]2+(alc) + 4 Cl- (aq) ↔ CoCl42-(alc) + 6 H2O(l)
Adding water would would increase [H2O] in the (alc) causing a Le Châtelier shift ← to form more reactants.
[Co(H2O)6]2+(alc) + 4 Cl- (aq) ← CoCl42-(alc) + 6 H2O(l)↑
