Silver nitrate is a clear crystal. The silver nitrate as any nitrate will dissolve in water. Silver nitrate makes colorless solution.
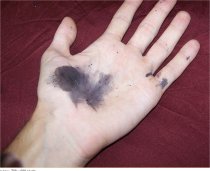
Silver nitrate will slowly react with skin to make a fine black silver metal powder that is very difficult to remove. Silver nitrate is classified as caustic but is only mildly so to skin and is even used as an antiseptic. If you work with silver nitrate without gloves, you very likely will stain your skin. |
|
||||||
Sodium chloride (table salt) will dissolve in water since all alkali metals are soluble and most halides are soluble too.
|
  |
||||||
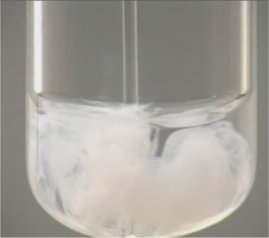
When the two solutions are mixed Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) The Ag+(aq) and Cl-(aq) ions attract one another to make a solid, a precipitate. Neither Na+nor NO3- ever form ppts. They are always left behind as spectator ions. Silver, merucry (I), and lead halide are important exceptions to the halide solubility rule.
The white precipitate AgCl(s) forms. The net ionic reaction is: Ag+(aq) + Cl-(aq) → AgCl(s) |
|||||||